Graphene oxide (GO) is a derivative of graphene, which itself is a single layer of carbon atoms arranged in a hexagonal lattice. Graphene has remarkable properties, such as exceptional strength, conductivity, and flexibility. However, graphene oxide is different due to the presence of oxygen-containing groups, which significantly alter its characteristics.
Structure and Composition
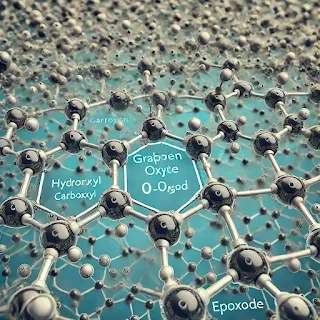
Graphene oxide consists of graphene sheets that have been functionalized with oxygen-containing groups such as hydroxyl (-OH), carboxyl (-COOH), and epoxide (-O-) groups. These functional groups attach to the graphene's carbon backbone and disrupt its perfect lattice structure. This makes GO more chemically reactive compared to pure graphene.
Synthesis
GO is typically synthesized using chemical oxidation methods, one of the most common being the Hummers' method. This involves treating graphite (the raw material) with strong acids and oxidizers, such as sulfuric acid, sodium nitrate, and potassium permanganate, which introduce the oxygen groups into the carbon structure.
Properties
Electrical Conductivity: While graphene is an excellent conductor, GO is an electrical insulator due to the disruption in the π-conjugation of carbon atoms by the oxygen groups. However, GO can be reduced (by removing some oxygen) to form reduced graphene oxide (rGO), which partially restores its conductivity.
Mechanical Strength: GO retains some of graphene’s mechanical strength but is generally weaker due to the defects introduced by oxygen. However, it is still incredibly strong compared to many materials and can form tough, lightweight composites.
Solubility and Dispersion: The oxygen groups make GO hydrophilic (water-attracting), allowing it to be easily dispersed in water and other solvents. This makes it easier to handle in many chemical processes than pure graphene, which is hydrophobic (water-repelling).
Chemical Reactivity: The oxygen functional groups make GO highly chemically reactive. This allows it to be modified and functionalized for various applications, such as drug delivery or sensors.
Applications
Graphene oxide's unique properties have led to a range of potential applications in different fields:
Biomedical Applications: GO is being researched for use in drug delivery, bioimaging, and biosensors. Its ability to disperse in water and interact with biomolecules makes it a good candidate for targeting diseases like cancer.
Electronics: Though GO is not conductive, reduced GO (rGO) can restore some of graphene’s conductivity, and the combination of these materials is being explored in flexible electronics, supercapacitors, and energy storage devices.
Water Filtration: GO membranes have shown promise in filtering water at the molecular level. The spaces between GO sheets can be controlled to allow the passage of water while blocking larger contaminants like salts and organic molecules.
Composites and Materials Science: GO is used to reinforce polymers, creating lightweight, strong materials that can be used in aerospace, automotive, and other industries where strength and weight are critical factors.
Energy Storage: Due to its large surface area and electrochemical properties, GO and rGO are being explored for use in batteries (e.g., lithium-ion batteries) and supercapacitors.
Toxicity and Environmental Concerns
The safety of graphene oxide in biological systems is a topic of ongoing research. Some studies suggest that GO can cause toxicity in living cells under certain conditions, potentially due to its sharp edges or oxidative properties. However, other studies indicate that it can be biocompatible, depending on the dose and the form of GO used. Further research is necessary to fully understand its long-term effects on health and the environment.
Reduction of Graphene Oxide
GO can be reduced back to a form similar to graphene, known as reduced graphene oxide (rGO), through chemical, thermal, or electrochemical methods. rGO has improved electrical conductivity compared to GO, though it may not fully regain the properties of pristine graphene due to residual defects from the oxidation process.
Challenges
Controlling Properties: The properties of GO can vary widely depending on how it is synthesized. Controlling these properties consistently remains a challenge for industrial-scale applications.
Scalability: Although GO can be produced in larger quantities compared to pristine graphene, making it at an industrial scale with uniform quality remains difficult.
Safety: More research is needed to determine how GO interacts with biological systems and the environment, especially if used in consumer products or biomedical devices.
Conclusion
Graphene oxide is a versatile material with a wide range of potential applications due to its tunable properties. Its water solubility, reactivity, and ability to form composites make it a valuable tool in materials science, energy storage, electronics, and biomedical applications. However, challenges related to scalability, property control, and safety need to be addressed before it can be widely adopted in commercial applications.
However, to break it down by individual elements that make up graphene oxide:
Carbon (C):
- Protons: 6
- Neutrons: 6 (typically, for the most common isotope, carbon-12)
- Electrons: 6
Oxygen (O):
- Protons: 8
- Neutrons: 8 (typically, for the most common isotope, oxygen-16)
- Electrons: 8
Hydrogen (H) (present in small amounts in hydroxyl or carboxyl groups):
- Protons: 1
- Neutrons: 0 (for the most common isotope)
- Electrons: 1
The exact number of each of these particles in a sample of graphene oxide depends on the ratio of carbon, oxygen, and hydrogen atoms, which is variable and depends on how the graphene oxide was synthesized and functionalized.
For example, if you have a graphene oxide structure with a general formula of CxOyHz, where x, y, and z are numbers representing the relative amounts of carbon, oxygen, and hydrogen, you could calculate the total number of protons, neutrons, and electrons by summing the contributions from each atom type.
To determine the exact numbers for a given sample, you'd need its precise chemical composition.

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.